Helium out of a tank drops in temp as expected.Hydrogen isn't practical to store as a liquid for end-user applications. (You would have a choice between continuously-operating cryogenic refrigeration apparatus - $$$ and constant energy use - or accept slow evaporation along with having a place for the evaporated hydrogen to go that won't cause an explosion.) The most practical way to store hydrogen for anything more than short-term use is as a pressurised gas (700 bar pressure). If the temperature drops from 27 C (300 K) to -3 C (270 K) then the pressure would drop to 630 bar (ideal gas law) ... still much more than any appliance would want, so it still has to go through a pressure regulator to drop the pressure to something that the downstream appliance (whatever it may be) can use.
An interesting oddity of hydrogen is that due to its low mass, the temperature goes UP when it expands through a flow restriction. The mechanical energy from the pressure gets converted to heat. That happens with all gases, but for gases other than hydrogen (maybe helium, not sure), the temperature drop due to expansion is more than the temperature increase due to the conversion of mechanical energy to heat. Hydrogen is so light that the temperature increase from the mechanical-energy losses is greater than the temperature drop due to expansion.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Any GTAM'ers own an electric vehicle?
- Thread starter PrivatePilot
- Start date
What a colossal joke. Has the shape (and funny doors) of a sports car, but you can't drive it. Family can't get in it. Someone with limited mobility can't get in or out. Doesn't look like it has much suspension travel.
There already is an ideal taxi ... a London cab. The new ones are already electric.
There already is an ideal taxi ... a London cab. The new ones are already electric.
LOL

 www.threads.net
www.threads.net

@Westenberg (@thisiswestenberg) on Threads
**** it. We don’t need to report on the Tesla Robocab Fuckfest. All we need to do is share their own literal disclaimer from the event: 🪦
 www.threads.net
www.threads.net
Loading…
www.caranddriver.com

Dirty Frank
Well-known member
And yet there are plenty of shills predicting tesla stock is about to blow up.What a colossal joke. Has the shape (and funny doors) of a sports car, but you can't drive it. Family can't get in it. Someone with limited mobility can't get in or out. Doesn't look like it has much suspension travel.
There already is an ideal taxi ... a London cab. The new ones are already electric.
People shorted Apple for years and years until they finally worked it out that yes 35% margins can be maintained 
Apple however had competent management all through that period of crazy growth and superb supply chain management and low manufacturing problems.
Tesla ?? not sure and certainly quality issues are seemingly unending. BYD seems to eating Tesla's lunch and the majors are all in it now - very competitive especially here in Australia with no tariffs. Tesla still gets the press and the hype but not all admiring.
Musk wanted to sell to Apple....Apple declined.....would have been interesting.
I'm pretty impressed with BYD despite the goofy full name. Partner still iffy but warming up now. Trying to get her to a test drive since we have a dealer in town.
Apple however had competent management all through that period of crazy growth and superb supply chain management and low manufacturing problems.
Tesla ?? not sure and certainly quality issues are seemingly unending. BYD seems to eating Tesla's lunch and the majors are all in it now - very competitive especially here in Australia with no tariffs. Tesla still gets the press and the hype but not all admiring.
Musk wanted to sell to Apple....Apple declined.....would have been interesting.
I'm pretty impressed with BYD despite the goofy full name. Partner still iffy but warming up now. Trying to get her to a test drive since we have a dealer in town.
TSLA took a hit today. Looks like investors weren't impressed.And yet there are plenty of shills predicting tesla stock is about to blow up.
And yet there are plenty of shills predicting tesla stock is about to blow up.
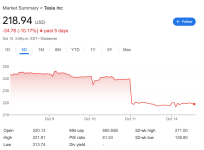
Yeah, that didn't work out so well for them, a 10% slaughter the day after the "robotaxi" event. It seems that the whole Musk reality distortion field of whatever comes out of his mouth being taken as gospel is finally wearing thin - the public and investors saw right through this whole sh!tshow and punished the stock immediately afterwards.
Between Tesla stock being priced as a "technology company" instead of the car company that it actually is, all the competition coming onboard building better cars all around, and Musk's behaviour turning off an increasingly larger percentage of his customer base day after day, the stock is in for a lot of pain in the years ahead. A lot of people are going to lose their shirt when the collapse comes, and I think it's coming inside the next 36 months.
The sooner Tesla removes Musk from the board and separates itself from his batshittery, the better.
495 pages & 7 years later, still hasn't pulled the trigger.
Bruh.
495 pages & 7 years later, still hasn't pulled the trigger.
Bruh.
Soon, very soon..,, several models in the pipeline in the next few years
Loading…
www.caranddriver.com
several models in the pipeline in the next few years
You still actually believe everything that comes out of Elons piehole, don't you?
That was about what Honda has in the pipeline, not Tesla.
Honda's aim to reduce weight and to keep the extra height to a minimum is commendable. Let's see what they come up with. I bought my Bolt because it was basically the smallest and lightest EV that had good range.
Honda's aim to reduce weight and to keep the extra height to a minimum is commendable. Let's see what they come up with. I bought my Bolt because it was basically the smallest and lightest EV that had good range.
That was about what Honda has in the pipeline, not Tesla.
Honda's aim to reduce weight and to keep the extra height to a minimum is commendable. Let's see what they come up with. I bought my Bolt because it was basically the smallest and lightest EV that had good range.
Not sure what we’ll buy next. I’d never buy a Tesla because of the dirty association to Musk and his batshittery now, and GM is kinda out as well after they nonsensically decided to abandon CarPlay on their EV’s moving forward.
I’m seriously eyeballing the Ioniq5, I love that design.
At this point I’m hoping my 2011 Volt will hang in for another 2ish years at which point we’ll grab a used Ioniq5 and I’ll inherit my wife’s 2017 Volt.
Not sure what we’ll buy next. I’d never buy a Tesla because of the dirty association to Musk and his batshittery now, and GM is kinda out as well after they nonsensically decided to abandon CarPlay on their EV’s moving forward.
I’m seriously eyeballing the Ioniq5, I love that design.
At this point I’m hoping my 2011 Volt will hang in for another 2ish years at which point we’ll grab a used Ioniq5 and I’ll inherit my wife’s 2017 Volt.
GM is making some curious decisions, why did they abandon the Ultium branding?
interestingly, the market is thirsty for a Honda EV, this bodes well for a "real" Honda EV in the future.
the Prologue is selling suprisingly well, its even out selling the Ioniq5 and gaining on the Mach E
Loading…
insideevs.com
So, at one point Elon bragged that there was 1.5 million pre-orders for the Cybertruck.
Estimates are that about 15,000 have been delivered.
Tesla announced recently that you can now order a non-foundation model CT for delivery in the next few weeks without a reservation.
Something doesn't add up here.
Estimates are that about 15,000 have been delivered.
Tesla announced recently that you can now order a non-foundation model CT for delivery in the next few weeks without a reservation.
Something doesn't add up here.
Sochi
Well-known member
It’s funny reading this discussion - with all the hate towards Elon let’s say everybody completely stop buying Teslas, so with Tesla accounted for just under 50% of total electric car market will it spell a complete disaster for the “electrification revolution” what is being pushed on us so hard these days?
And I am not hating on it, just watching from the sidelines for now…
And I am not hating on it, just watching from the sidelines for now…
which reminds meSo, at one point Elon bragged that there was 1.5 million pre-orders for the Cybertruck.
Estimates are that about 15,000 have been delivered.
Tesla announced recently that you can now order a non-foundation model CT for delivery in the next few weeks without a reservation.
Something doesn't add up here.
Total sales numbers for EVs have been steadily increasing in the North American market, even with Tesla's weakness.It’s funny reading this discussion - with all the hate towards Elon let’s say everybody completely stop buying Teslas, so with Tesla accounted for just under 50% of total electric car market will it spell a complete disaster for the “electrification revolution” what is being pushed on us so hard these days?
And I am not hating on it, just watching from the sidelines for now…

Electric Vehicle Sales Mark Another Record in Q3, Thanks to Higher Incentives, More Choices - Cox Automotive Inc.
Cox Automotive publishes a quarterly Kelley Blue Book new-vehicle sales report enumerating electric vehicle sales.















